Acids Bases and Salt notes
Properties of Acids:
– Produce hydrogen ions [H+] in H2O.
– Sour taste.
– Turn blue litmus red.
– Act as electrolytes in Solution.
– Neutralize solutions carrying hydroxide ions.
– React with several metals releasing Hydrogen gas.
– React with carbonates releasing CO2 (g)
– Destroy body tissues.
– corrode metal surface quickly.
On the basis of origin, acids are classified as :
a. Organic acids: Acids derived from living organisms like plants and animals . For
example: citric acid is present in fruits, acetic acid present in vinegar, oxalic acid
present in tomato, tartaric acid present in tamarind, lactic acid present in sour milk
and curd.
b. Mineral acids: They are also called inorganic acids. They are dangerous Example sulphuric acid (H2SO4), hydrochloric acid (HCl) etc.
➣ On the basis of their strength, acids are classified as :
a. Strong acids: Completely dissociate into its ions in aqueous solutions.
Example: Nitric acid (HNO3), sulphuric acid (H2SO4), hydrochloric acid (HCl).
b. Weak acids: Weak acids are those acids which do not completely dissociate into
its ions in aqueous solutions. For example: carbonic acid (H2CO), acetic acid
(CH3COOH).
➣ On the basis of their concentration, acids are classified as :
a. Dilute acids: Have a low concentration of acids in aqueous solutions.
b. Concentrated acids: Have a high concentration of acids in aqueous solutions.
➣ On the basis of number of hydrogen ion, acids can be classified as :
Monoprotic acid – Such type of acid produces one mole of H+ ions per mole of acid, e.g., HCl , HNO3
Diprotic acid – They can produce two moles of H+ ions per mole of acid, e.g., H2SO4.
Triprotic acid – They produce three moles of H+ ions per mole of acid, e.g., H3PO4.
Polyprotic – They can produce more than three H+ ions per mole of acid.
➣ Properties of Base:
– Produce hydroxide ions [OH –] in H2O.
– Water soluble bases are called alkalies.
– Bitter Taste
– Turn Red Litmus blue.
– Act as electrolytes in Solution.
– Neutralize solutions containing H+ ions.
– Have a slippery, ‘soapy’ feel.
– Dissolve fatty material.
➣ On the basis of their strength, bases are classified as:
a. Strong bases: Strong bases are those bases which completely dissociate into
its ions in aqueous solutions. Example: sodium hydroxide (NaOH), potassium
hydroxide (KOH).
b. Weak bases: Weak bases are those bases which do not completely dissociate
into its ions in aqueous solutions. For example: ammonium hydroxide (NH4OH).
➣ On the basis of their concentration, bases are classified as:
a. Dilute bases: Have a low concentration of alkali in aqueous solutions.
b. Concentrated bases: Have a high concentration of alkali in aqueous solutions.
➣ Strength Of Acid Or Base Solutions:
A scale for measuring hydrogen ion concentration in a solution,
called pH scale has been developed. The p in pH stands for ‘potenz’ in
German, meaning power.
p= potential or Power H = Hydrogen
| pH =7 | Neutral Solution | H3O+ = OH – |
| pH>7 | Basic Solution | H3O+ < OH – |
| pH<7 | Acidic Solution | H3O+ > OH – |
Range of pH is from 1 to 14
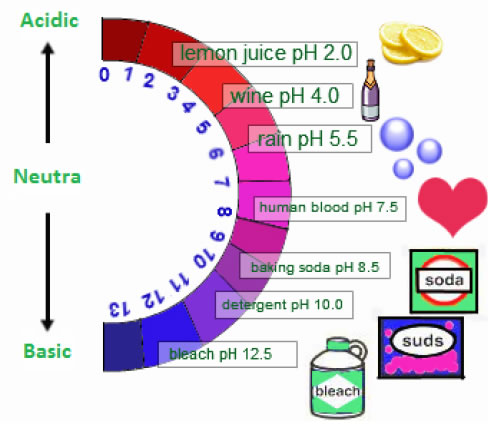
➣ pH Sensitivity of Plants & Animals:
• Human body works in a narrow range of pH 7 to 7.8. Acidity can be lethal for plants and animals.
• pH of Digestive System: Stomach secretes HCl to kill bacteria in the
food. The inner lining of stomach protects vital cells from this acidic
pH.
• pH and tooth decay: Lower pH because of sour food and sweet food can
cause tooth decay. The pH of mouth should always be more than 5.5.
• pH as self defense mechanism in plants & animals: Certain
animals like bee and plants like nettle secrete highly acidic substance
for self defense.
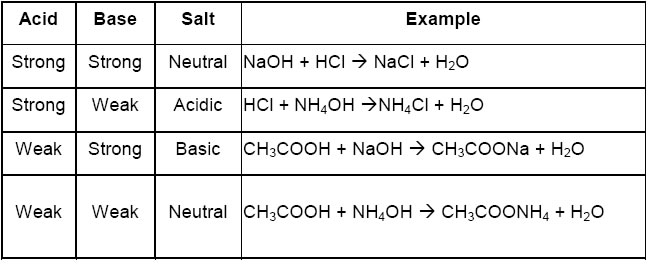
➣ Properties of salts:
• Salts form by the combination of acid and base through neutralization reaction.
• The acidic and basic nature of salts depends on the acid and base combined in neutralization reaction.
• The most common salt is sodium chloride or table salt which forms by
the combination of sodium hydroxide (base) and hydrochloric acid.
• Other examples include Epsom salts (MgSO4) used in bath salts, ammonium nitrate (NH4NO3
) used as fertilizer, and baking soda (NaHCO3) used in cooking.
• The pH of salts solution depends on the strength of acids and base combined in neutralization reaction.
• Indicators – Indicators are substances which indicate the acidic or basic nature of the solution by their colour change.
The colour of some acid – base indicators in acidic and basic medium are
given below :
S. No. |
Indicators |
Colour in |
Colour in |
acidic medium |
basic medium |
||
1 |
Litmus solution |
Red |
Blue |
2 |
Methyl Orange |
Pink |
Orange |
3 |
Phenolphthalein |
Colourless |
Pink |
4 |
Methyl red |
Yellow |
Red |
Chemical properties of acids:
i) Acids react with active metals to give hydrogen gas.
Zn + H2SO3 →ZnSO4 + H2
ii) Acids react with metal carbonate and metal hydrogen carbonate to give carbon dioxide.
NaHCO3 +HCl → NaCl + H2O + CO2
iii) Acids react with bases to give salt and water. This reaction is called as neutralization reaction.NaOH + HCl → NaCl +H2O
iv) Acids react with metals oxides to give salt and water.
CuO + H2SO4 → CuSO4 + H2O
Addition of Acids or Bases to Water
The process of dissolving an acid, specially nitric acid or sulfuric
acid or a base in water is a highly exothermic one. As a rule:
Always add acid to water and never the other way! The acid must be
added slowly to water with constant stirring. If one mixes the other
way by adding water to a concentrated acid, the heat generated causes
the mixture to splash out and cause burns.
➣ Chemical properties of Bases:
i) Reaction with Metals - Certain reactive metals such as Zinc,
Aluminium, and Tin react with alkali solutions on heating and hydrogen
gas is evolved. 2NaOH + Zn → Na2ZnO2
+H2
ii) Reaction with acids -Bases react with acids to form salt and water. KOH +HCl → KCl +H2O
iii) Reaction with Non -metallic oxides – These oxides are generally acidic in nature. They react with bases to form salt and water. 2NaOH + CO2 → Na2CO3 + H2O
➣ Some Important Chemical Compounds:•
• Common Salt (NaCl)
Sodium chloride is known as common salt. Its main source is sea
water. It is also exists in the form of rocks and is called rock
salt.
Common salt is an important component of our food. It is also used for
preparing sodium hydroxide, baking soda, washing soda etc.
• Sodium hydroxide (NaOH)
Prepared by Chlor Alkali process :Electricity is passed through an
aqueous solution of Sodium chloride (called brine). Sodium chloride
decomposes to form sodium hydroxide. Chlorine gas is formed at the
anode, and hydrogen gas at the cathode. Sodium hydroxide solution is
formed near the cathode.
2NaCl(aq) + 2 H2O (l) → 2NaOH(aq) + Cl2(g) + H2(g)
• Bleaching powder:
Bleaching powder is represented as CaOCl2, though the actual composition is quite complex.
Bleaching powder is produced by the action of chlorine on dry slaked lime. Ca(OH)2 + Cl2 → CaOCl2+ H2O
• Baking soda:
Sodium hydrogen carbonate (NaHCO3) Preparation: NaCl + H2O + CO2+ NH3 → NH4Cl + NaHCO3
•Washing soda: Sodium carbonate N2CO3.10H2 In the first step, sodium carbonate is obtained by heating baking soda. 2 NaHCO3(heat) →Na2CO3 + H2O + CO2
Then washing sod
a is produced by recrystallisation of sodium carbonate Na2CO3 + 10H2O → Na2CO3 .10H2O
• Plaster of Paris:
Calcium sulphate hemihydrate CaSO4. ½ H2O Prepared by heating Gypsum at 373K. CaSO4. 2H2O(Heat at 373K) → CaSO4. ½ H2O + 1½ H2O
This is mind map
It is the url for youtube video of this chapter : https://youtu.be/dNIFzaUNFxk
Thanks for visiting
plz follow this page


No comments:
Post a Comment